Quantitative Proteomic Services
BGI Genomics supports multiple workflows for proteomic quantitation. We have developed innovative solutions for optimizing the process of accurately quantifying proteins in complex biological matrices and other complex mixtures. Our quantitative services include Label-Free Data-Independent Acquisition (DIA), Isobaric Label (iTRAQ, TMT and IBT tag), Rapid DDA Proteomics, Targeted Proteomics (PRM), Nanoproteomics, FFPE Proteomics and Metaproteomics.
We are happy to work with you to determine the best quantitative proteomics service for your project1.
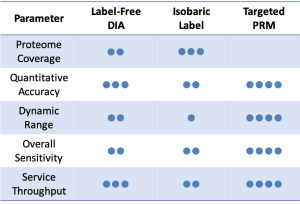
Data-Independent Acquisition (DIA) is a label-free quantitative technique which provides highly consistent quantitation and broad proteome coverage. We create a customized spectral library with your specific sample and then analyze individual samples using nano-flow LC-MS/MS with DIA scanning mode2.
Label-Free DIA Quantitative Proteomics service is ideal for long-term projects or projects with large sample sets which require accurate and reproducible quantitation.
Isobaric Label services incorporate sample multiplexing to provide deep proteome coverage and highly precise quantitation of small or medium-sized sample sets3.
BGI Genomics provides Isobaric Label Quantitative Proteomics service using iTRAQ (Isobaric Tags for Relative and Absolute Quantitation), IBT (Isobaric Tags for Quantitation) and TMT (Tandem Mass Tags for Quantitation) technologies developed by AB SCIEX, BGI Genomics and Thermo Scientific, respectively.
In contrast to label-dependent quantitative proteomics such as the iTRAQ method, label-free data-dependent acquisition (DDA) proteomics doesn’t need to use expensive a stable isotope for labeling of proteins. This allows accurate protein quantification of samples without additional error-prone in vitro labelling reactions and enables fast proteome identification and quantification4.
Traditional target protein analysis is based on antibody techniques, which have limitations in throughput and specificity. However, parallel reaction monitoring (PRM) approaches5 is able to resolve these obstacles.
As the gold standard for targeted proteomics research based on MS platforms, PRM technology can quickly and accurately determine dozens of proteins simultaneously in complex biological samples and solve slow verification problems.
Nanoproteomics refers to quantitative proteomics analysis of small cell populations (typically < 5,000 cells) by the combination of in-situ cleavage and DDA label free technology, and offers possibilities for analysis unavailable with conventional protein extraction and mass spectrometry6.
BGI Genomics has extensive experience in the field of nanoproteomics spanning cellular heterogeneity research across rare cell populations, hard-to-obtain clinical specimens, and pathological tissues.
FFPE sample refers to the tissue sample treated with FFPE method, which can be stored steadily for decades at room temperature and is the gold standard for histoppathological analysis7. BGI Genomics has extensive experience in the field of FFPE proteomics of tumor heterogeneity research.
Since its inception in 2004, metaproteomics aims at the large-scale characterization of all proteins expressed by environmental microbiota at a given point in time, which can better promote the in-depth study of the diversity, structure and potential gene function of the microbial community8.
BGI Genomics has extensive experience in the field of metaproteomics spanning microorganisms research across human fecal samples, water samples, and fermentation liquor samples.
Reference
[1] Cheung, C.H.Y. and H.F. Juan, Quantitative proteomics in lung cancer. J Biomed Sci, 2017. 24(1): p. 37.
[2] Searle, B.C., et al., Chromatogram libraries improve peptide detection and quantification by data independent acquisition mass spectrometry. Nat Commun, 2018. 9(1): p. 5128.
[3] Rauniyar, N. and J.R. Yates, 3rd, Isobaric labeling-based relative quantification in shotgun proteomics. J Proteome Res, 2014. 13(12): p. 5293-309.
[4] Krasny L, et al., Data-independent acquisition mass spectrometry (DIA-MS) for proteomic applications in oncology. Mol Omics, 2020. 9(4).
[5] Method of the Year 2012. Nat Methods, 2013. 10(1).
[6] L. Yi, et al., Advances in microscale separations towards nanoproteomics applications, 2017.1523: 40–48.
[7] Marchione D M, et al., HYPERsol: High-quality data from archival FFPE tissue for clinical proteomics. J Proteome Res, 2020 Feb 7; 19(2): 973–983.
[8] Kleiner M. Metaproteomics: much more than measuring gene expression in microbial communities. MSystems, 2019 May-Jun; 4(3): e00115-19.
TOP