New Classification of Gastrointestinal Stromal Tumors Guides Precision Treatment | Nature Communications
2024-11-04

A recent study offering a comprehensive view of the genome and transcriptome of gastrointestinal stromal tumors (GISTs) has introduced a new, multi-omics-based molecular classification of these tumors. This research not only categorizes GISTs into specific molecular subtypes but also identifies YLPM1, a potential tumor suppressor gene, providing new insights into GIST pathogenesis and laying the groundwork for precision treatment.
Conducted by the Institute of Intelligent Medical Research (IIMR) at BGI Genomics in collaboration with the Shanghai Institute of Nutrition and Health, University of Chinese Academy of Sciences, Ren Ji Hospital, Shanghai Jiao Tong University, and others, the study was published in Nature Communications in November 2024. The findings bring clarity to the complex nature of GISTs, identifying critical genetic signatures that contribute to varying levels of tumor aggressiveness and treatment response.
GISTs, the most common type of sarcoma, display a fascinating range of aggressiveness. Unlike other sarcomas, GISTs can vary from small, benign tumors to highly invasive, metastatic cancers. While many GISTs share common mutations in KIT or PDGFRA, their clinical behavior varies significantly. This study sheds light on these differences, revealing genetic signatures that influence how these tumors evolve and respond to treatment, offering a roadmap for more targeted therapies.
Complex genomic characteristics of GISTs
A key finding of the study is that GISTs exhibit remarkably low rates of somatic coding mutations—one of the lowest observed among human cancers. However, they accumulate other genomic alterations, including copy number variations (CNVs) and structural variants (SVs), which contribute to their increased aggressiveness.
Alterations in genes such as CDKN2A, DEPDC5, RB1, and DMD are more frequent in advanced GISTs, and massive genomic rearrangement events like chromothripsis and kataegis contribute to tumor progression by restructuring the genome. These mutations play a critical role in transforming GISTs into more invasive forms, highlighting the need for targeted interventions in advanced cases.
Adding another layer of complexity, GISTs exhibit significant genetic heterogeneity, with different mutations occurring across various tumor sites, especially in metastatic cases. This diversity complicates treatment strategies, as therapies like tyrosine kinase inhibitors (TKIs), commonly used for KIT-mutant GISTs, may become less effective over time as the tumor adapts and develops resistance. Understanding the genetic diversity within an individual’s GIST tumors can aid in refining treatment plans and exploring combination therapies to counter resistance.
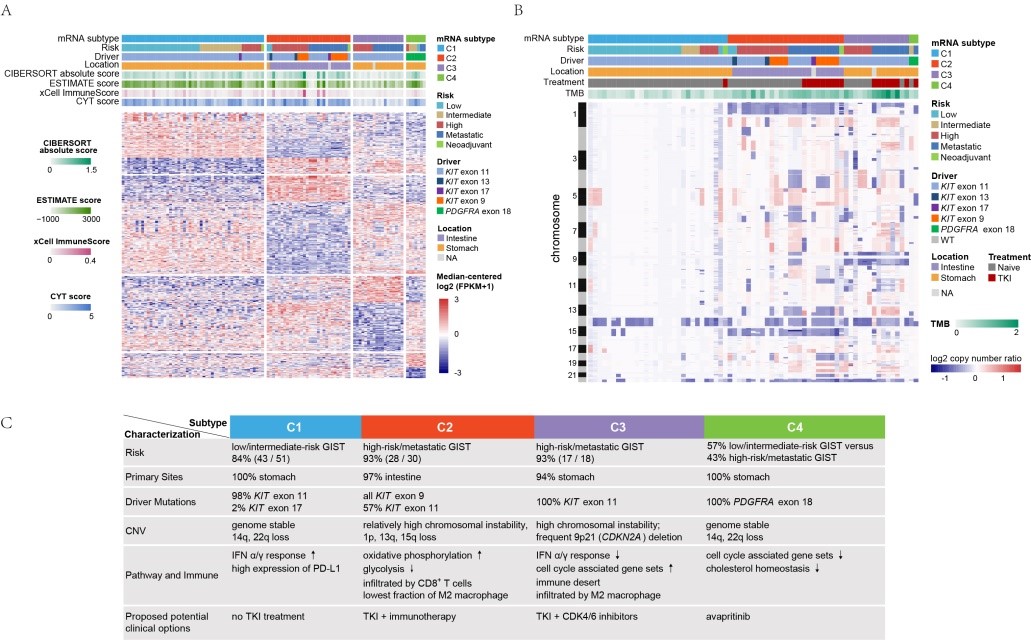
Figure: Genomic, expression profiles, immunological and clinical characteristics of different GIST subtypes and potential treatment strategies
The study's transcriptome-based classification reveals four distinct molecular subtypes within GISTs, each with unique genomic and immune characteristics that can inform treatment strategies. For instance, C1, a genome-stable subtype primarily consisting of low- to intermediate-risk gastric GISTs with KIT mutations, generally has a favorable prognosis with surgical resection alone. The C2 subtype, defined as CD8+ inflamed, comprises high-risk intestinal GISTs with high infiltration of immune cells, especially CD8+ T cells, suggesting that these tumors may benefit from a combination of TKI therapy and immunotherapy.
Meanwhile, the C3 subtype, known as the immune desert subtype, represents high-risk gastric GISTs with frequent CDKN2A deletions. It shows limited immune activity and potential responsiveness to CDK4/6 inhibitors combined with TKIs. Lastly, the C4 subtype includes PDGFRA-mutant GISTs, which respond well to PDGFRA inhibitors like avapritinib, though they remain resistant to standard TKIs.
Novel Tumor Suppressor Gene in GISTs
Another significant finding of the study is the identification of YLPM1 as a GIST-specific tumor suppressor gene. Although broadly expressed across various tissues, YLPM1 appears particularly crucial in GISTs, where its inactivation promotes cell proliferation and increases oxidative phosphorylation, fueling tumor growth. In experimental models, restoring YLPM1 function in GIST cells slowed tumor progression, positioning it as a promising target for future therapies. This finding offers new directions for treatment, especially in targeting YLPM1-deficient GISTs to limit their growth.
This integrative multi-omics analysis not only advances our understanding of the molecular profile of GISTs but also bridges the gap between basic research and clinical application. The study empowers physicians to consider more personalized treatment strategies by identifying specific molecular subtypes. For example, patients with C2 tumors might benefit from a combination of TKI and immunotherapy, while those with C3 tumors could explore the synergistic effects of CDK4/6 inhibitors with TKIs.
The study’s insights underscore the importance of understanding the genetic nuances within GISTs and adapting treatment approaches accordingly. As clinical trials further validate these findings, this molecular classification could revolutionize how GISTs are managed, bringing us closer to a future of personalized, precision medicine for GIST patients.
About BGI Genomics
BGI Genomics, headquartered in Shenzhen, China, is the world's leading integrated solutions provider of precision medicine. Our services cover more than 100 countries and regions, involving more than 2,300 medical institutions. In July 2017, as a subsidiary of BGI Group, BGI Genomics (300676.SZ) was officially listed on the Shenzhen Stock Exchange
Read more:
New Combined Therapy Shows Promise for Advanced Stomach Cancer Treatment | Nature Communications
Largest Multi-Omics Study Unveils Prognostic Genetic Insights in Colorectal Cancer
Research reveals: Gut bacterium Megamonas tied to obesity risk | BGI Insight