Novel NIPT-based Method for Detecting Copy Number Variations in the DMD Gene | BGI Insight
2024-07-16

A recent large cohort study, conducted jointly by researchers from the Nanjing Women and Children’s Healthcare Hospital and BGI Genomics, implemented a self-developed prenatal screening strategy to effectively identify and classify 128 true-positive exonic copy number variations (CNVs) in the Duchenne muscular dystrophy (DMD) gene using NIPT data. By reporting 33 different pathogenic or likely pathogenic maternal CNVs in the DMD gene, this study fills a significant information gap regarding the frequency and spectrum of maternal CNV carriers in the Chinese population.
Discover 33 Unique Pathogenic CNVs
The researchers re-analyzed 135,047 NIPT samples collected from Nanjing Women and Children’s Healthcare Hospital and Suzhou Municipal Hospital between January 2017 and December 2021. They identified a total of 224 maternal CNVs in the DMD gene, of which 128 were confirmed as true-positive exonic CNVs. Among these, 48 had specific exons refined, and five large pathogenic or likely pathogenic CNVs were validated using chromosomal microarray analysis (CMA).
Pathogenic and Recurrent CNVs Located
Among the 128 true-positive CNVs, 64 were classified as pathogenic or likely pathogenic, 40 as uncertain significance (VUS) variants, and 24 as likely benign. Notably, 33 of the pathogenic or likely pathogenic CNVs were found in the DMD gene, with some appearing repeatedly in specific exons. Additionally, 12 of the 33 unique pathogenic CNVs were recurrent, most frequently found in exons 48–51, followed by exons 51–52, exons 45–55, and exons 49–51. Using data from the Leiden Open Variation Database, researchers were able to predict potential phenotypes for these CNVs.
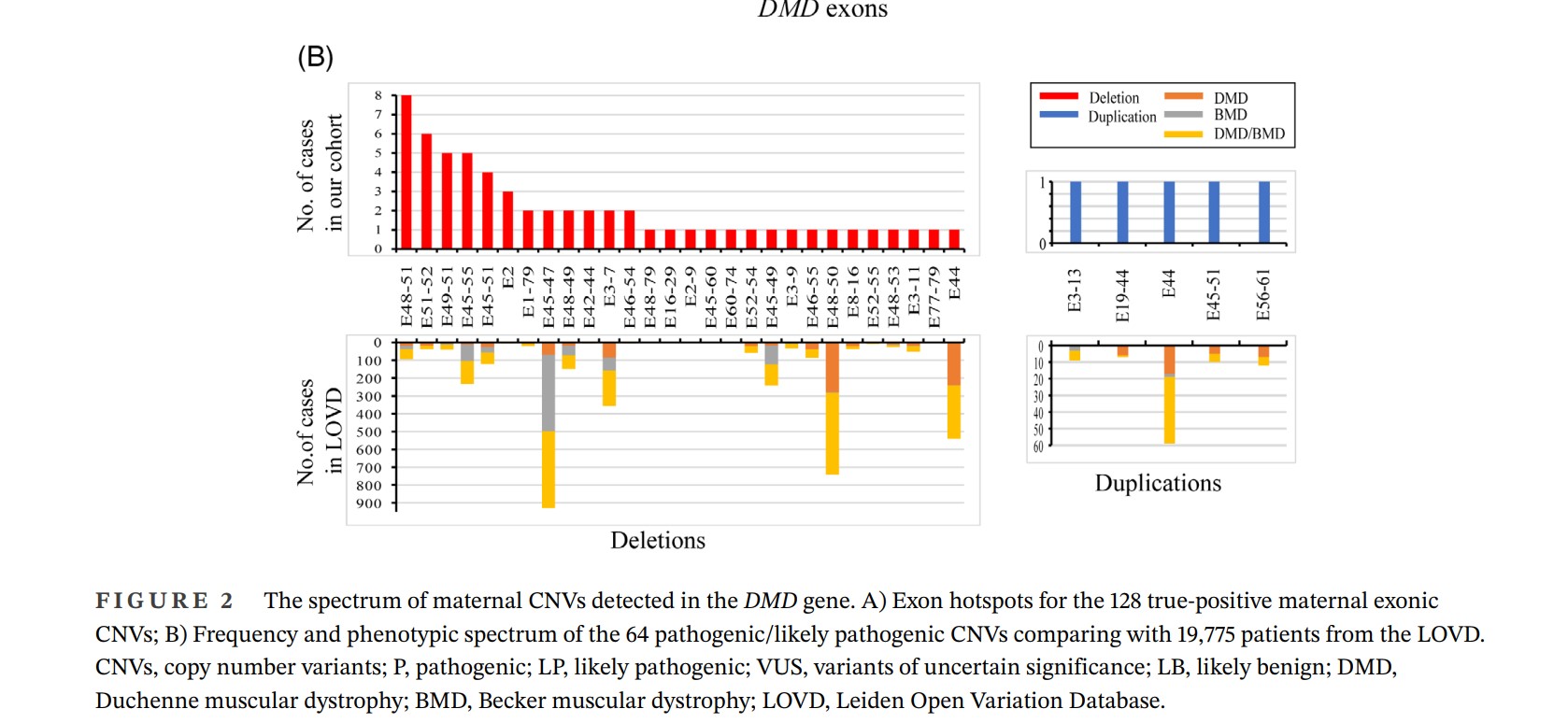
Gene Deletion Hinders Development
The study also included follow-ups on 7 out of 11 male offspring, aged between 8 months and 5 years, to investigate their serum creatine kinase (CK) levels and potential genetic conditions. Elevated CK levels were found in 71.43% of these boys, indicating possible muscle issues.
A typical out-of-frame deletion in exons 48-50 of the DMD gene was discovered in one case. Despite appearing healthy at one year old, the son had a significantly high CK level (12,416.8 U/L), suggesting a potential diagnosis of Duchenne Muscular Dystrophy (DMD).
Another case involved a rare out-of-frame deletion in exons 2-9. At two and a half years old, this boy showed normal motor skills and CK levels (125.4 U/L) but delayed speech development and minor behavioral issues. This deletion is linked to neurodevelopmental disorders rather than DMD, as previously observed in monozygotic twins with similar genetic profiles.
Global Relevance Guiding Future Research
Researchers have noted carrier frequencies of DMD gene variants in various populations, including Belgium (1/2612), the United States (1/717), and Israel (1/1046), highlighting the global relevance of this genetic research. This study emphasizes the need for more research and long-term monitoring to understand the full impact of these genetic deletions. By leveraging existing NIPT data, the research introduces a cost-effective method for prenatal screening of CNVs in the DMD gene, expanding the practical applications of NIPT without requiring extra investment.
Read more:
Enhancing Non-Invasive Early Detection of Gestational Diabetes with cfDNA Sequencing
Enhancing Prenatal Screening with Low-Pass Genome Sequencing | BGI Insight