Research Proves Stool DNA as Non-Invasive Alternative for Colorectal Cancer Screening | BGI Insight
2024-11-05

A recent prospective cross-sectional study in Thailand demonstrates that multitarget stool DNA testing is highly sensitive and specific for detecting colorectal cancer (CRC) among Thai individuals. Researchers believe that this testing method could serve as a viable non-invasive alternative to colonoscopy, especially in settings where colonoscopy is less accessible or less accepted by patients.
This study was conducted by BGI Genomics in 2023, in collaboration with Professor Varut Lohsiriwat’s team from the Faculty of Medicine, Siriraj Hospital, Mahidol University, Thailand. The research team included Professor Varut Lohsiriwat, Dr. Aitsariya Mongkhonsupphawan (M.D, Ph.D, Faculty of Medicine, Siriraj Hospital, Mahidol University), and Pornraksa Ovartchaiyapong (Lecturer, Faculty of Medicine, Siriraj Hospital, Mahidol University). The study was published in the Asian Pacific Journal of Cancer Prevention (APJCP) in October 2024.
Researchers focused on evaluating the diagnostic performance of the multitarget stool DNA testing for detecting CRC and advanced adenoma, using colonoscopy as the reference standard. The study included both asymptomatic and symptomatic patients who underwent stool DNA testing followed by colonoscopy. The multitarget stool DNA test targeted methylation statuses of SDC2, ADHFE1, and PPP2R5C genes. Sensitivity, specificity, and other diagnostic parameters were analyzed.
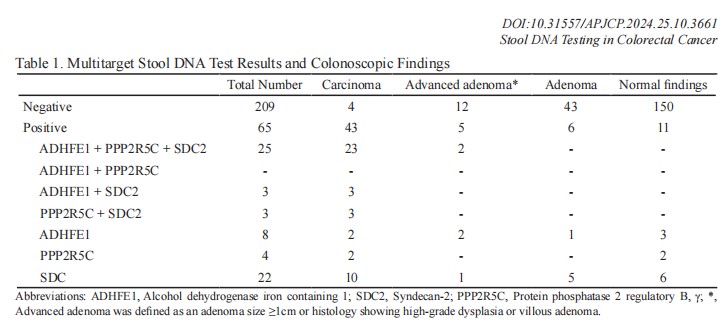
Figure: Multitarget stool DNA testing results vs. colonoscopy results
In the study, data from 274 patients (mean age 62 years, with a predominantly female cohort at 60.6%) were evaluated. Colorectal cancer was identified in 17.2% of participants, while 6.2% were found to have advanced adenomas, which are precursors to cancer. The multitarget stool DNA test, a non-invasive screening method, yielded impressive results: it detected CRC with a sensitivity of 91.5% and a specificity of 90.3%, accurately identifying cancer in over 90% of cases. The test's sensitivity was consistent across both right-sided and left-sided colon lesions, at 92.3% and 91.2%, respectively.
For advanced colorectal neoplasms, which include both CRC and advanced adenomas, the test demonstrated a sensitivity of 75% and a specificity of 91.9%, marking it as a promising tool for the early detection of serious colorectal conditions. These findings highlight the test's potential as an effective screening option for detecting CRC, especially for larger lesions, although some limitations remain for detecting smaller-sized abnormalities.
BGI Genomics’ COLOTECT® stool DNA testing kits were used for sample and raw data collection. COLOTECT® is a non-invasive tool for early colorectal cancer detection based on DNA methylation testing, targeting the methylation profile of colorectal exfoliated cells to assess the risk of colorectal cancer and advanced adenomas. This tool requires no special equipment, imposes no dietary restrictions, and is entirely non-invasive. In recent years, DNA methylation-based colorectal cancer detection has gained recognition, being incorporated into multiple CRC screening guidelines and expert consensus worldwide.
The study results indicate that multitarget stool DNA testing is highly sensitive and specific for CRC detection among Thai individuals. This testing could provide a viable non-invasive alternative to colonoscopy, especially in settings where colonoscopy is less accessible or less accepted by patients.

In June 2024, to advance the global understanding of CRC and explore ways to close the awareness gap, BGI Genomics invited Prof. Varut Lohsiriwat from Mahidol University and Dr. Zhu Shida from BGI Genomics to share insights after reviewing the BGI Genomics 2024 Global State of Colorectal Cancer Awareness Report.
As early as 2022, BGI Genomics Southeast Asia team established a close partnership with Professor Varut Lohsiriwat from the Faculty of Medicine, Siriraj Hospital, Mahidol University, inviting him to participate in the BGI Genomics overseas science education program “Your Health, Our Concern.” In this program, Professor Lohsiriwat shared valuable knowledge and insights on colorectal cancer treatment.
With support from global experts, such as Professor Varut’s team, and advanced genetic sequencing technology like BGI Genomics’ COLOTECT® Stool DNA Methylation Test, which extracts DNA from intestinal exfoliated cells in stool samples and detects aberrant methylation in genes related to CRC (SDC2, ADHFE1, and PPP2R5C) through fluorescent PCR, we expect to significantly reduce the global burden of CRC.
About COLOTECT®
COLOTECT® is a non-invasive fecal DNA test developed by BGI Genomics for detecting CRC and precancerous lesions. It uses multiplex methylation-specific PCR (MSP) technology to trace abnormal DNA-methylation biomarkers in CRC from stool samples. COLOTECT® has a sensitivity of 88% for detecting CRC, and a sensitivity of 46% for the early detection of advanced adenomas—both of which outperform conventional fecal tests.
About BGI Genomics
BGI Genomics, headquartered in Shenzhen, China, is the world's leading integrated solutions provider of precision medicine. Our services cover more than 100 countries and regions, involving more than 2,300 medical institutions. In July 2017, as a subsidiary of BGI Group, BGI Genomics (300676.SZ) was officially listed on the Shenzhen Stock Exchange.
Read more:
BGI Genomics COLOTECT 3.0 Demonstrates Higher Colorectal Cancer Detection Sensitivity than FIT
2024 Global State of Colorectal Cancer Awareness Report
Beating Colorectal Cancer with Early Screening and Detection: Patient's Voice
Largest Multi-Omics Study Unveils Prognostic Genetic Insights in Colorectal Cancer
Will Colorectal Cancer Become a Chronic Disease? | BGI Insight